In our great-great-grandparents’ time, the average life expectancy at birth was 47 years. This was not because people died at that age, but because infant mortality was the order of the day. Anyone who went into an operating theatre had just a one in three chance of survival. Nearly a quarter of deaths in a country like the US were due to bacterial infections. A visit to the dentist, a sore throat or any small wound could have fatal consequences, and often an infection could only be contained by amputation. Such was the past without antibiotics, until the use of penicillin in the 1940s changed everything—but unfortunately, only temporarily. Today the very serious problem of so-called antibiotic-resistant superbugs, described as an “overlooked pandemic” and one of the ten greatest threats to global health, presents us with the chilling prospect of a return to the world of our great-great-grandparents, a world without weapons against bacterial infections.
Alexander Fleming’s discovery of penicillin in 1928 and its subsequent development by the team of Howard Florey and Ernst Boris Chain have been hailed as the greatest triumph in the history of medicine. Nowadays, the overuse of the term “revolutionary discovery” has almost rendered it meaningless, but no other is more deserving. In the golden age of antibiotics, from the 1950s to the 1980s, life expectancy increased to 78 years, and bacterial diseases were no longer a concern for people in developed countries. The then US Surgeon General William H. Stewart is often quoted as saying in 1967 that it was “time to close the books on infectious diseases and declare the war against pestilence won.” And while Stewart apparently never said any such thing, the phrase reflects a view that was common at the time.
Third leading global cause of death
But multi-resistant bacteria have been getting in the way of that dream future. For years, antibiotic resistance has been viewed as “an abstract risk to health, a potential cause of illness and death at some point in the future. This way of thinking makes it easy to ignore,” warned an editorial in The Lancet in January 2022, adding that “the new comprehensive estimates show that antimicrobial resistance is killing large numbers of people now.” The editorial was prompted by the publication of the results of the most comprehensive study to date, GRAM (Global Research on Antimicrobial Resistance), led by the University of Oxford and the Institute for Health Metrics and Evaluation (IHME) at the University of Washington in Seattle.
The GRAM results, which refer to 2019, reveal that antimicrobial resistance was associated with 4.95 million deaths worldwide that year, 1.27 million of them directly caused by resistant bacteria. GRAM collects data from 204 countries and territories on 23 types of bacteria and 88 specific resistances. The bacterium causing the most deaths was a common gut microbe, Escherichia coli, while the most lethal specific resistance was Staphylococcus aureus to the antibiotic methicillin. The data place antimicrobial resistance as the third leading cause of death globally if all associated deaths are counted, or twelfth, almost on a par with AIDS and malaria combined, if directly caused deaths are considered.
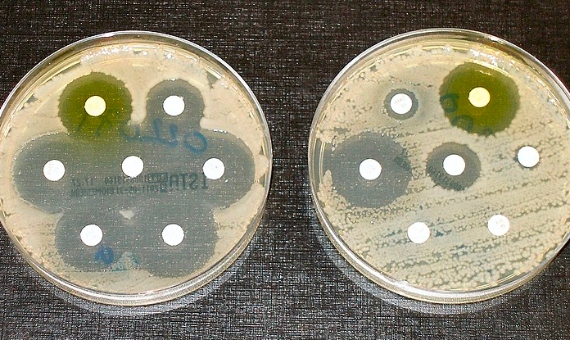
So far GRAM has only published data for 2019, but as study co-leader Mohsen Naghavi of IHME tells OpenMind, they are currently working on analysing the trend over time. However, other studies have shown that the problem of resistance is getting worse. In 2019, a report by the United Nations, the World Health Organization and others estimated that, at the current rate, deaths from resistance could rise to 10 million a year globally by 2050.
This is all the more alarming given that this is by no means a new or unforeseen problem, but is so old that Fleming himself warned in 1945, when he received the Nobel Prize, that the overuse of antibiotics could encourage the proliferation of resistant microbes. In fact, the problem of resistance is as old as life itself; it should be made clear that antibiotics are not necessarily the cause of resistance, but that resistance arises in nature through random genetic mutations.
Bacterial resistance, a numbers game
A good example of this is the work of Ivan Erill at the University of Maryland, Baltimore County and his collaborators at the Universitat Autònoma de Barcelona in Spain. In 2019, they discovered that resistance to sulphonamides—antibacterials used before antibiotics—arose in nature more than 500 million years ago. Unlike antibiotics, which are produced by or derived from fungi, sulphonamides are synthetic: humans invented them. And yet mutant versions of the enzyme attacked by these drugs are almost as old as the bacteria themselves. “Resistance predates the existence of the antimicrobials themselves,” Erill tells OpenMind. As the bioinformatician explains, “it’s a numbers game.” With an estimated bacterial population of five million trillion trillion, he says, the genetic diversity is so immense that “it is almost a given that somewhere there is a bacterium resistant to any drug you can possibly imagine.”
However, once these resistances emerge, which Erill says for any new antibiotic will appear “within years, if not months,” antibiotic use does lead to increased growth of resistant versus sensitive strains, as well as the exchange of resistance-carrying genes between bacteria, in a kind of evolution on demand in response to the pressure from the antibiotic drugs. “With selective pressure on, it takes little time to accumulate enough mutations for full resistance,” says Erill.
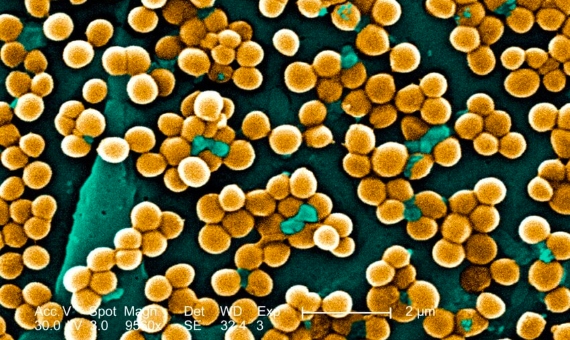
For all these reasons, the experts call for more investment, effort and international public and private collaboration to establish global mechanisms to fight antibiotic resistance, learning from the world’s effective and rapid response to COVID-19. The GRAM authors point to five main courses of action: prevention and control of infections, vaccination, moderating the use of antibiotics (including in animal husbandry), eliminating their use when they are not necessary—such as in viral infections—and developing new antibiotics.
As for the first two strategies, which seek to prevent infections in the first place, for the GRAM authors they are undoubtedly the most essential. “It is common sense that the most important prevention of infectious death is vaccination and improving risk factors related to hygiene,” says Naghavi. Mitigating these risks also includes water sanitation and waste management infrastructure where they are deficient. As for vaccines, they currently only exist against one of the six most dangerous bacteria, Streptococcus pneumoniae.
Restricting the use of antibiotics and the risk of anti-COVID measures
Regarding the use of antibiotics, there is generally more control and awareness in developed countries than in areas of the developing world, where they are often sold without prescription or regulation and consumed in greater quantities. Experts warn especially of the risk of expired drugs and incomplete treatments; in these situations, a sub-optimal level of active ingredient is particularly likely to encourage the growth of less sensitive strains.
However, livestock farming is an unresolved issue given that 66% of antibiotics are used in farm animals, not people. These drugs enter the food chain and are released into the environment, polluting the water, in addition to the wastewater discharges coming from drug production plants. In 2017, Michael Gillings of Macquarie University in Australia and colleagues at the Chinese Academy of Sciences revealed that China’s river estuaries contain up to 100 million antibiotic resistance genes per gram of sediment. According to Gillings, this is equivalent to one million resistance genes in the head of a matchstick. “Every environmental compartment investigated has resistance genes,” he explains to OpenMind. Not all of those present in the environment are the result of pollution, he says, but many of the naturally occurring ones are transferred to pathogenic bacteria, and can even spread through the air. Therefore, the antibiotic pollution is coupled with that of the resistance genes themselves in the environment.

The COVID-19 pandemic has also contributed to the problem. “Most coronavirus patients are also receiving antibiotics for treatment of secondary bacterial infections,” University of Westminster microbiologist Manal Mohammed tells OpenMind. “This massive rise in antibiotics will undoubtedly contribute to the rise of antibiotic resistant bacteria.” But COVID-19 has also increased another risk factor, the overuse of disinfectants such as hand gels and antibacterial products. Research prior to the pandemic had already revealed the emergence of bacterial strains that are resistant, or rather tolerant, to many of the compounds in these products, including alcohol itself.
A 2016 study found 48% resistance in E.coli and 64% in Pseudomonas aeruginosa to 25 different brands of hand gels. How bacteria learned to tolerate these compounds is not yet known in detail; it has been suggested that such substances stimulate pumping systems in bacterial cell membranes that eject them outwards— like a ship’s pump ejects water—or that they induce DNA damage that can lead to resistance mutations. It has also been suggested that certain disinfectants stimulate DNA exchange between bacteria, which may spread resistance genes. Whatever the mechanism, experts are warning that the current overuse of disinfectants will only make the problem worse.
Next-generation antibiotics and vaccines
The development of new antibiotics is a particularly critical issue. Since the last of these drugs emerged in the 1980s, there has been what is known as a discovery void, decades without any new discoveries. The rapid spread of resistance “creates an incentive problem for the pharma industry,” says Erill. In other words, companies do not see a return on investment in new antibiotics. “To me, the problem is that pharma is still thinking of this in terms of an obsolete business model.” They want to pump them out in droves and sell them “by the tonne, not the microgram,” he says. Even in these cases, he adds, generics will eliminate the profit. For Erill, possible solutions include public research or market regulation, not only by restricting use, but also by setting prices that ensure profitability.
In the meantime, science is exploring alternative avenues. “Vaccines are a potential answer, but require government research monies,” says Gillings. Another possibility is the use of bacteria-killing viruses, called bacteriophages or phages. This approach emerged in the early 20th century, but was all but abandoned with the discovery of antibiotics. Interest has been revived in recent times and is now the focus of studies by numerous groups, including that of Manal Mohammed. One drawback is that bacteria also become resistant to phages, but the combination of different weapons can be key: when a certain bacterium becomes resistant to a phage, it does so by shedding the capsule that the virus exploits to attack it, but the disappearance of this capsule makes the bacterium vulnerable to an antibiotic to which it was previously resistant.

In short, big problems call for big solutions. In an article in The Conversation, Manchester University emeritus professor Mukesh Kapila, a global health expert, urged that the crisis of antimicrobial resistance should be tackled with “a dedicated organisation with the universal legitimacy of a UN body, political clout of a G20, deep pockets of a global fund, brainpower of a space agency, campaigning zeal of an NGO, mould-breaking power of a social movement, and leveraging capacity of a public-private partnership.”
Comments on this publication