Until the day arrives when the vaccine against SARS-CoV-2 is finally available in a vial, our collective hopes are pinned on the development of possible treatments. This area contains so many unknowns that science is advancing in fits and starts, and a drug that looks promising today may be discredited in a few weeks, even more so considering that nearly 1,500 clinical trials worldwide are actively searching for therapies for the new coronavirus. Without losing sight of this volatility, here we review the main therapeutic approaches to date at the forefront of finding a cure for COVID-19.
Plasma from people recovering from COVID-19
Since the 19th century, when scientists first began to experiment with the immunization of animals with microbes in order to extract the serum and treat the sick, this passive immunization —a treatment with antibodies produced by another human or animal— has endured as a rapid strategy against infectious diseases. The first results in China against the new coronavirus were promising, and there are now several programs underway in various countries, including a large project in the U.S. led by infectious disease expert Arthur Casadevall at Johns Hopkins University, which brings together 40 institutions.

This approach can be taken further by concentrating and purifying the SARS-CoV-2 antibodies in the plasma from numerous patients, creating what is known as hyperimmune globulin (H-Ig). Unlike raw plasma, which is used directly from the source after being screened for possible pathogens, the H-Ig technique provides a stable and standardized drug. Several companies are pursuing this line of research, including an international alliance called the CoVIg-19 Plasma Alliance.
Recombinant antibodies against the virus
A natural continuation of the above is to apply genetic engineering techniques (or recombinant DNA) to obtain antibodies against the virus. Some companies opt for a large-scale attack by introducing into animals or cell cultures certain human genes that are capable of producing a full repertoire of different antibodies to SARS-CoV-2, known as polyclonals. CSL Behring, SAb Biotherapeutics and GigaGen are exploring this option under different strategies.
The last step in the antibody therapies is also obtained by genetic technologies, but directed exclusively against a specific part of the virus; these are known as monoclonal antibodies (MAb). Detailed knowledge of the structure of the Spike (S) protein, which SARS-CoV-2 uses to invade human cells, has made it possible to hunt for MAb capable of blocking the infection. However, new drugs can take a long time to develop and must also undergo a cycle of clinical trials that often lasts several years.
Remdesivir
Following that thread, it becomes clear that the best candidates for developing treatments during the pandemic emergency will be those drugs that already exist, and especially those that are approved for other indications and can be repositioned to fight against COVID-19. Numerous clinical trials are testing compounds that already existed before the pandemic, some of which are already approved for use against other diseases and others that are still in the experimental phase.

Among the latter is remdesivir, an antiviral medication developed in 2009 by Gilead Sciences that failed to show efficacy for its original purposes, so it is not yet approved for any medical conditions. Preliminary reports regarding its effectiveness against COVID-19 suggested a possible benefit. A later study in China questioned its usefulness, but a clinical trial with more than 1,000 patients has shown that remdesivir shortens recovery time from 15 days to 11 days compared to a placebo, reducing mortality from 12% to 7%. Remdesivir inhibits the RNA polymerase, an enzyme the virus uses to produce its proteins.
Favilavir (favipiravir, avifavir)
Like remdesivir, other compounds also act on the virus’ RNA polymerase. One of them is EIDD-2801, which the pharmaceutical giant Merck has unveiled as its showpiece antiviral drug against the new coronavirus. Also in this category is favipiravir or favilavir, created by the Japanese group Fujifilm, marketed under the name Avigan and approved in that country against the flu. Since 2019, it has been sold as a generic medicine and in February 2020 it was authorized in China as a treatment against COVID-19. Russia has also recently approved its use under the name avifavir. The effectiveness of this compound against SARS-CoV-2 has been demonstrated in vitro, and preliminary clinical results indicate that it may shorten the time needed to clear the virus from the body. Several clinical trials are attempting to evaluate its effectiveness in more detail.
Chloroquine and hydroxychloroquine
If one drug has been in the spotlight during this pandemic, it is hydroxychloroquine (along with its precursor, chloroquine), a classic medication used against malaria. Early results showed an inhibition of cellular SARS-CoV-2 infection in vitro and possible benefits in patients. Chloroquine was included in the World Health Organization’s (WHO) “Solidarity” clinical mega-trial, aimed at testing the four most promising potential treatments, and the use of this drug has been endorsed by figures such as President Donald Trump. The possibility that a cheap and widely available drug could successfully fight against the scourge of the coronavirus was received with enormous hope around the world.
However, researchers soon found a lack of conclusive evidence, and the initial studies were questioned. One of them, led by French microbiologist Didier Raoult, is under investigation by the journal that published it, while another, still in preprint without formal publication, has been withdrawn pending a review.
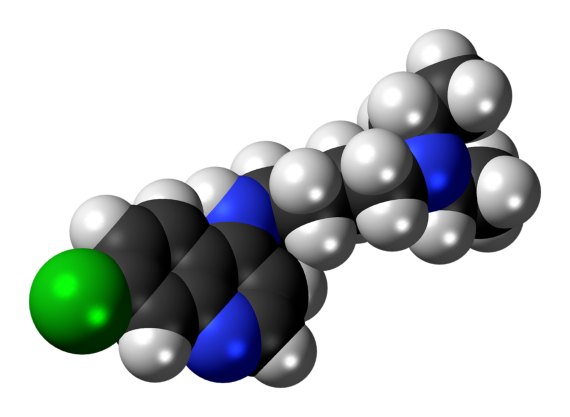
The final nail in chloroquine’s coffin seemed to come from a large observational study in The Lancet that has gathered data from more than 96,000 patients, and according to which chloroquine would not only provide no benefit, but could increase mortality, while another large review also failed to confirm initial hopes.
In view of the new data, the WHO has decided to temporarily suspend its trials with this drug. But in recent days, a new plot twist has added to the confusion. Several researchers have questioned the validity of The Lancet study, including the data itself, procured by a data analytics company. In view of the inconsistencies, which led to more than 200 researchers signing a letter criticizing the study, The Lancet has placed the study in quarantine until its validity is established. The same data analytics company also provided the material for a study on the effectiveness of the antiparasitic drug ivermectin against COVID-19, which is now also under scrutiny. The chloroquine soap opera will no doubt continue.
Cytokine storm inhibitors
Part of the pathology associated with COVID-19 lies in an overreaction of the immune system, something already observed in other infections. In a number of patients, the infection triggers a hyperinflammatory immune response brought about by the so-called Cytokine Release Syndrome, or more popularly known as a cytokine storm. Although it is not yet clear to what extent this may be a widespread problem among patients, several researchers are testing drugs already known to be able to calm this hyperimmune response. Much of the interest has focused on tocilizumab, a monoclonal antibody that inhibits the action of the immune mediator interleukin-6 (IL-6).
Although favourable results have been shown with the use of tocilizumab, it is still early to know if this drug will be proposed as a possible effective therapy against COVID-19; a recent study warns of an increase in secondary bacterial infections in treated patients, which is not surprising when one of the body’s natural immune mechanisms is inhibited.
Comments on this publication